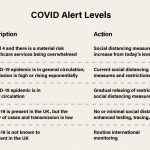
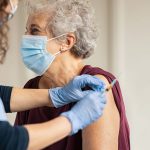
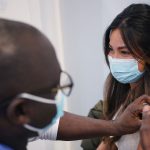
Two COVID-19 vaccines are currently being administered in the UK – but there are several more that have been ordered and are yet to be approved.
The Pfizer-BioNTech jab got the UK’s COVID-19 vaccine programme off to a start on 8 December after being given emergency approval by the Medicines and Healthcare products Regulatory Agency (MHRA) less than a week before.
On 4 January, the Oxford-AstraZeneca jab also started being administered after approval on 30 December.
Only one other vaccine has been approved since but the government has ordered 300 million doses of five other vaccines.
These are the ones that have yet to be rolled out or approved:
Approved: Moderna – 17m doses ordered
The Moderna vaccine was approved on 7 January and is expected to be rolled out in April when it is sent over from the US.
It is an mRNA vaccine, like the Pfizer jab, and trials have found it to be 95% effective in preventing COVID-19.
Not approved: Novavax – 60m doses ordered
The Novavax vaccine is awaiting approval from the MHRA, which is expected imminently but is already being manufactured in England.
Novavax, an American vaccine development company that also has facilities in Sweden, started developing a COVID vaccine in January 2020.
It is a protein subunit vaccine that contains the spike protein of the COVID virus.
In January 2021, its phase three trials found it had 89.3% efficacy and provided strong immunity against new variants, including the Kent one.
Its vaccine is being made at Fujifilm’s site in Billington, Stockton-on-Tees and will undergo its “fill and finish” at a GlaxoSmithKline (GSK) facility in Barnard Castle, County Durham.
Not approved: Janssen (Johnson & Johnson) – 30m doses ordered
Only one jab is needed of this viral vector vaccine – the same type of technology as AstraZeneca’s.
Please use Chrome browser for a more accessible video player
It was approved in the US in February and the EU in March after trials found it was 67% effective at preventing COVID-19 and completely effective at preventing hospital admissions and death from the virus.
Janssen Pharmaceuticals is a Belgian subsidiary of pharma giant Johnson & Johnson, so the jab is being referred to by both names.
Not approved: Valneva – 100m doses ordered
The French pharmaceutical company’s jab is still in trials but manufacturing has begun at a site in West Lothian, Scotland.
It uses inactive whole virus technology – a dead version of the coronavirus that cannot cause disease but should teach the body’s immune system how to fight it.
If it is approved, 60m doses are expected to be administered in the second half of 2021 and the remaining 40m next year in case boosters are needed.
Not approved: GlaxoSmithKline/Sanofi Pasteur – 60m doses ordered
Trials had to belayed in December after phase one and two results found a low immune response in adults over 49.
They were restarted in February with more volunteers, including older adults, and if the data is positive it could move to late-stage trials in the second quarter of 2021.
The UK drugs giant and its French partner, which is the largest company devoted entirely to vaccines in the world, have said a vaccine could be available by the final quarter of 2021.
Please use Chrome browser for a more accessible video player
Not approved: CureVac – 50m doses ordered
An mRNA vaccine, like Pfizer’s and Moderna’s, the German company is in phase three trials.
It is also working with the UK government and GSK to develop a new variety of vaccine that can protect against multiple new COVID strains in one jab.
After phase one trials, the company said its first-generation vaccine closely mimics the immune response after natural COVID-19 infection.
A specialist in RNA technology for the past 20 years, CureVac joined the UK Vaccines Taskforce in February.
CureVac’s deal with the UK includes being able to manufacture its vaccines within the UK.