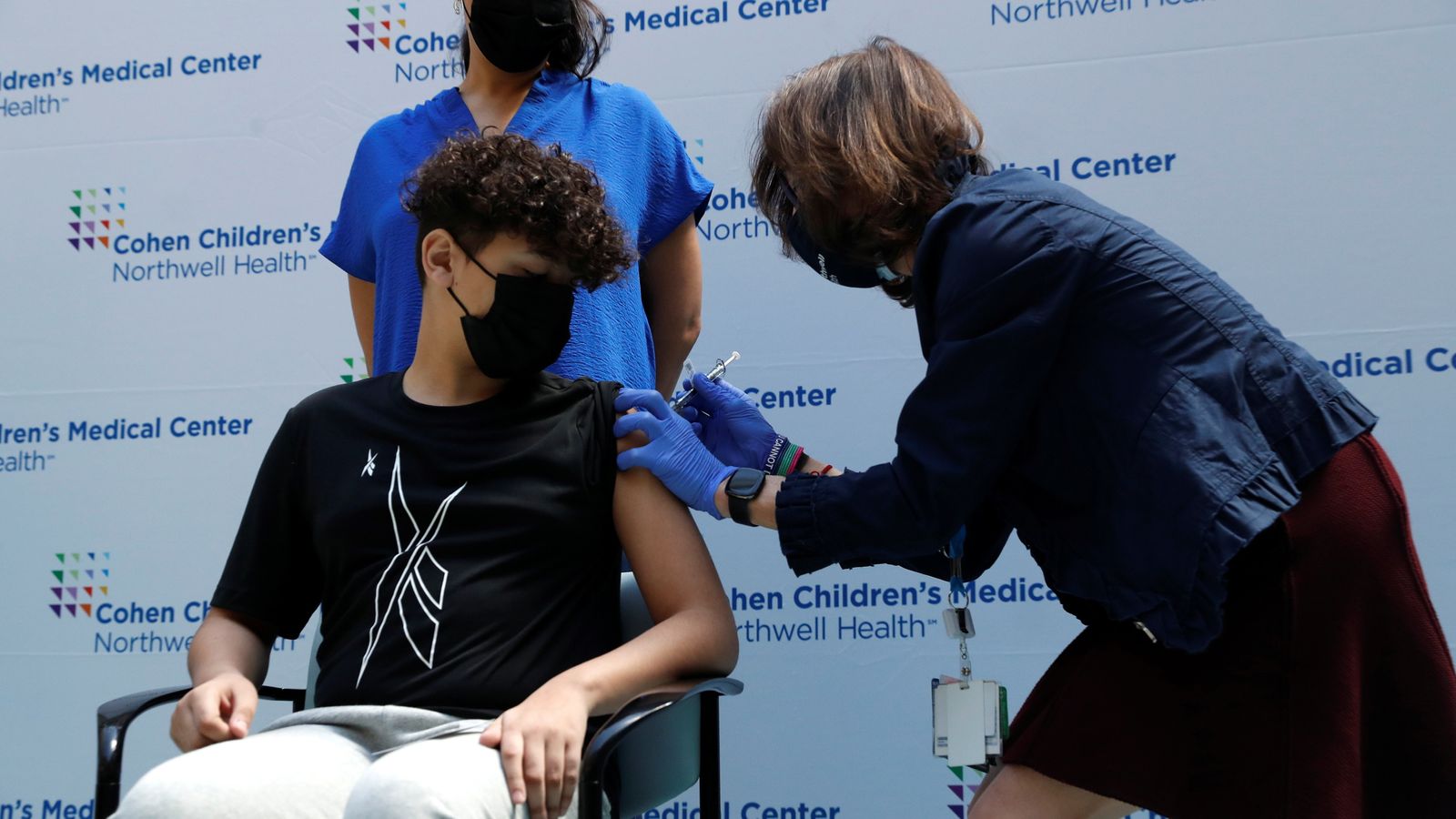
The UK’s medicines regulator has approved the Pfizer/BioNTech coronavirus vaccine for 12 to 15-year-olds.
The Medicines and Healthcare products Regulatory Agency (MHRA) said the decision followed a “rigorous review” of safety and effectiveness in that age group – and that the benefits of having the COVID jab outweighed the risks.
Dr June Raine, MHRA chief executive, said: “We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved COVID-19 vaccines and this surveillance will include the 12- to 15-year age group.”
Live COVID updates from the UK and around the world
A Department of Health and Social Care spokesperson said the next step was for the Joint Committee on Vaccination and Immunisation (JCVI) to advise whether routine vaccination should be offered to those aged 12 to 17.
“We will be guided by the expert advisors and will update in due course.”
The Pfizer/BioNTech jab was approved for use in the UK for 16 and 17 year olds in December 2020.
The JCVI’s current advice is that those aged 16 – 18 should be offered vaccination if they are in a priority Phase 1 group or they are the household contacts of someone who is immunosuppressed.
There is no routine vaccination of under 18s currently under way.
More than 2,000 children were involved in the clinical trial to determine the safety of the Pfizer/BioNTech vaccine, the chairman of the Commission on Human Medicines said.
Professor Sir Munir Pirmohamed said based on the data, the vaccine’s benefits “do outweigh any risk”.
He added out of the 2,000 children studied as part of the randomised, placebo-controlled clinical trials, there were no cases of COVID-19 from seven days after the second dose, compared with 16 cases in the placebo group.
In addition, data on neutralising antibodies showed the vaccine working at the same level as seen in adults aged 16-25 years.
“These are extremely positive results,” he said.