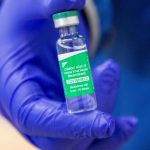
The UK has become the first country to approve a “game-changing” anti-viral pill that can be taken at home to treat COVID-19.
Molnupiravir can be taken by those who have tested positive and have at least one risk factor for developing severe illness, such as obesity, being over 60, diabetes or heart disease.
After promising trial results, the Medicines and Healthcare products Regulatory Agency (MHRA) has said it is safe and effective at reducing the risk of hospital admission and death in people with mild to moderate COVID who are at extra risk from the virus.
Developed by Ridgeback Biotherapeutics and Merck Sharp & Dohme (MSD), the drug works by interfering with the virus’s replication.
It inhibits COVID-19 from multiplying, keeping levels low in the body and ultimately reducing the severity of the disease.
The drug should be taken as soon as possible following a positive test and within the first five days, the MHRA advises.
COVID-19: REACT-1 study shows infections are at highest ever recorded level as researchers warn next 10 days will be ‘critical’
COVID-19: UK reports another 41,299 coronavirus cases and 217 deaths
COVID-19: Professor Jonathan Van-Tam warns of ‘potentially problematic’ Christmas and ‘hard months to come’
Last month, the government announced it had secured 480,000 courses of molnupiravir after a study showed it reduced the rate of hospital admissions and deaths by 50% in patients with mild to moderate symptoms.
The tablet was given twice a day to recently diagnosed patients, and the trial results made it one of the most promising drug developments of the pandemic so far.
Health Secretary Sajid Javid said: “Today is a historic day for our country, as the UK is now the first country in the world to approve an anti-viral that can be taken at home for COVID-19.
“This will be a gamechanger for the most vulnerable and the immunosuppressed, who will soon be able to receive the ground-breaking treatment.”
He added: “We are working at pace across the government and with the NHS to set out plans to deploy molnupiravir to patients through a national study as soon as possible.”
Follow the Daily podcast on Apple Podcasts, Google Podcasts, Spotify, Spreaker
Dr June Raine, MHRA chief executive, said the body is “satisfied” with molnupiravir being declared safe and effective for those at risk of developing severe COVID-19 disease and has granted its approval.
“Lagevrio (molnupiravir) is another therapeutic to add to our armoury against COVID-19,” she said.
“It is also the world’s first approved anti-viral for this disease that can be taken by mouth rather than administered intravenously.
“This is important, because it means it can be administered outside of a hospital setting, before COVID-19 has progressed to a severe stage.”
It comes as an estimated 1.2 million people in private households in the UK reported experiencing long COVID in the four weeks to 2 October, according to data from the Office for National Statistics (ONS).