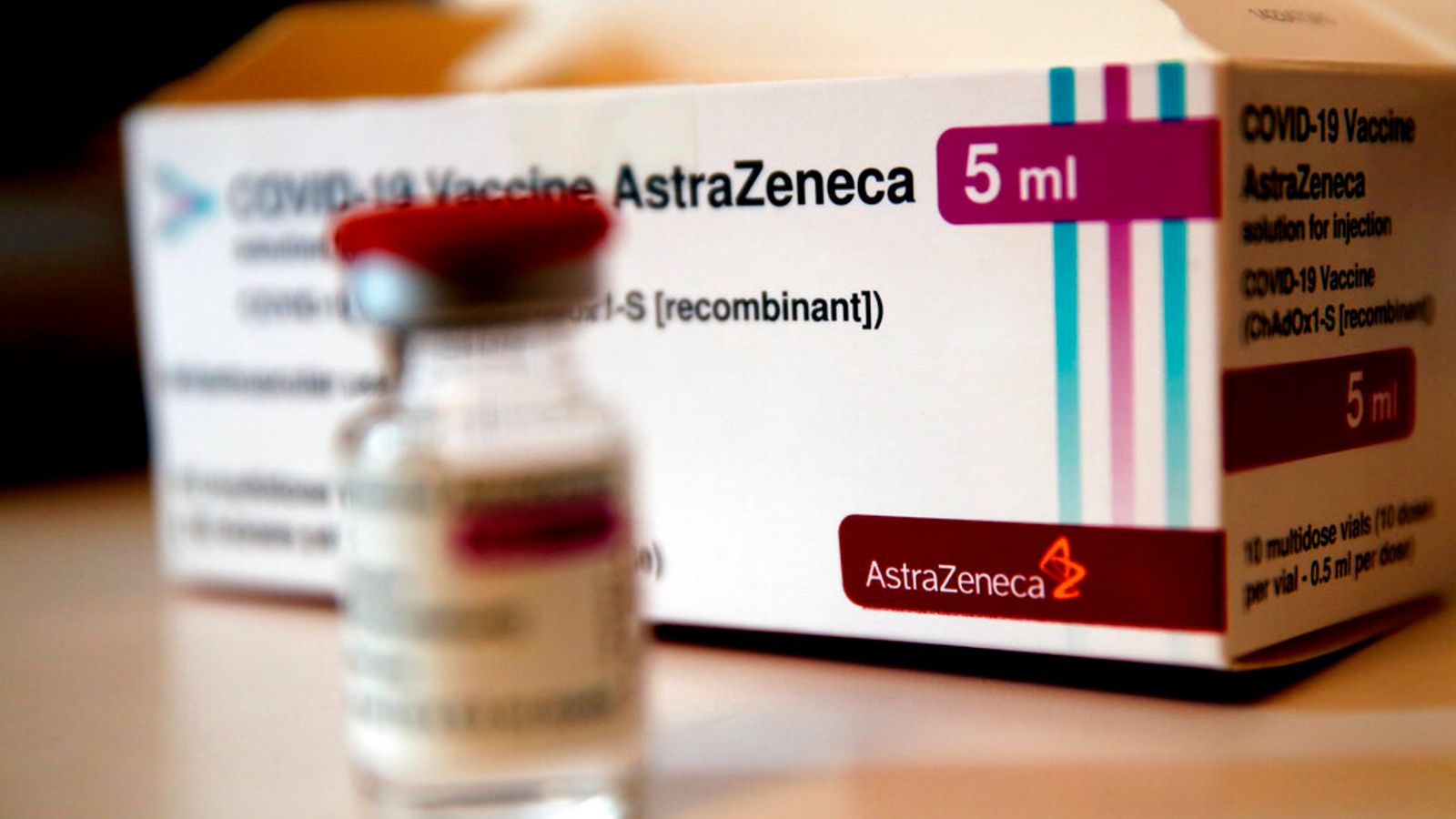
Thirty blood clotting cases were reported in the UK after the first 18 million doses of the Oxford-AstraZeneca vaccine, the medicines regulator has said.
The figure is 25 more than previously reported.
The count was updated as part of a rolling review into Britain’s COVID vaccines, with the Medicines and Healthcare products Regulatory Agency stressing the benefits of the jab “far outweigh any known side effects”.
Concerns have been raised about blood clots after a tiny proportion of cases among the tens of millions who have received the Oxford-AstraZeneca jab.
Some countries, such as Germany, have restricted its use to certain ages but the European medicines watchdog and the World Health Organisation both say it’s safe and effective.
The MHRA said on Thursday there had been “22 reports of cerebral venous sinus thrombosis (CVST) and 8 reports of other thrombosis events with low platelets”.
The figures cover 9 December 2020 to 21 March this year, when 15.8 million doses of the AstraZeneca vaccine had been administered, and around 2.2 million second doses.
The regulator stressed that all medicines have potential side effects and that the benefits of both COVID jabs means people should not hesitate to get vaccinated.
“The number and nature of suspected adverse reactions reported so far are not unusual in comparison to other types of routinely used vaccines,” said the MHRA report.
Its said the most common side effects reported for both the Pfizer and Oxford-AstraZeneca vaccines were “injection-site reactions” such as a sore arm, and general flu-like symptoms like headache, chills, aching, fatigue and nausea.
These are normal reactions to any vaccine as the body produces an immune response are should disappear after a day or two, said the MHRA.
It said the side effects are reported more frequently in younger people.
More than 31 million people in the UK have had a first dose of one of the two vaccines, with second doses topping first doses in recent days as older people get called up to complete their treatment.
A third approved vaccine, from Moderna, is also expected to be rolled out in April.
The vaccine rollout in the EU has been much slower and was hampered by some countries pausing the Oxford-AstraZeneca jab until the European regulator gave it the green light and concluded it did not increase the overall number of clots in the population.
Despite that, Germany said this week that it would not give the vaccine to under-60s on the recommendation of its national regulator.