A new drug has been found to slow the progression of Alzheimer’s, with experts hailing it as a “turning point” in the fight against the disease.
Donanemab was found to slow “clinical decline” by up to 35%, allowing people with Alzheimer’s to continue performing day-to-day tasks such as shopping, housekeeping, managing their finances and taking medication.
Following the findings of a trial of the drug, Alzheimer’s Research UK said “we’re entering a new era” where the disease “could become treatable”.
The health spending watchdog in England, the National Institute for Health and Care Excellence (NICE), is already assessing whether the drug can be used in the NHS.
Meanwhile, Alzheimer’s Society said treatments such as donanemab could one day mean the disease is comparable to long-term conditions such as asthma or diabetes.
The charity believes this “could be the beginning of the end for Alzheimer’s disease”.
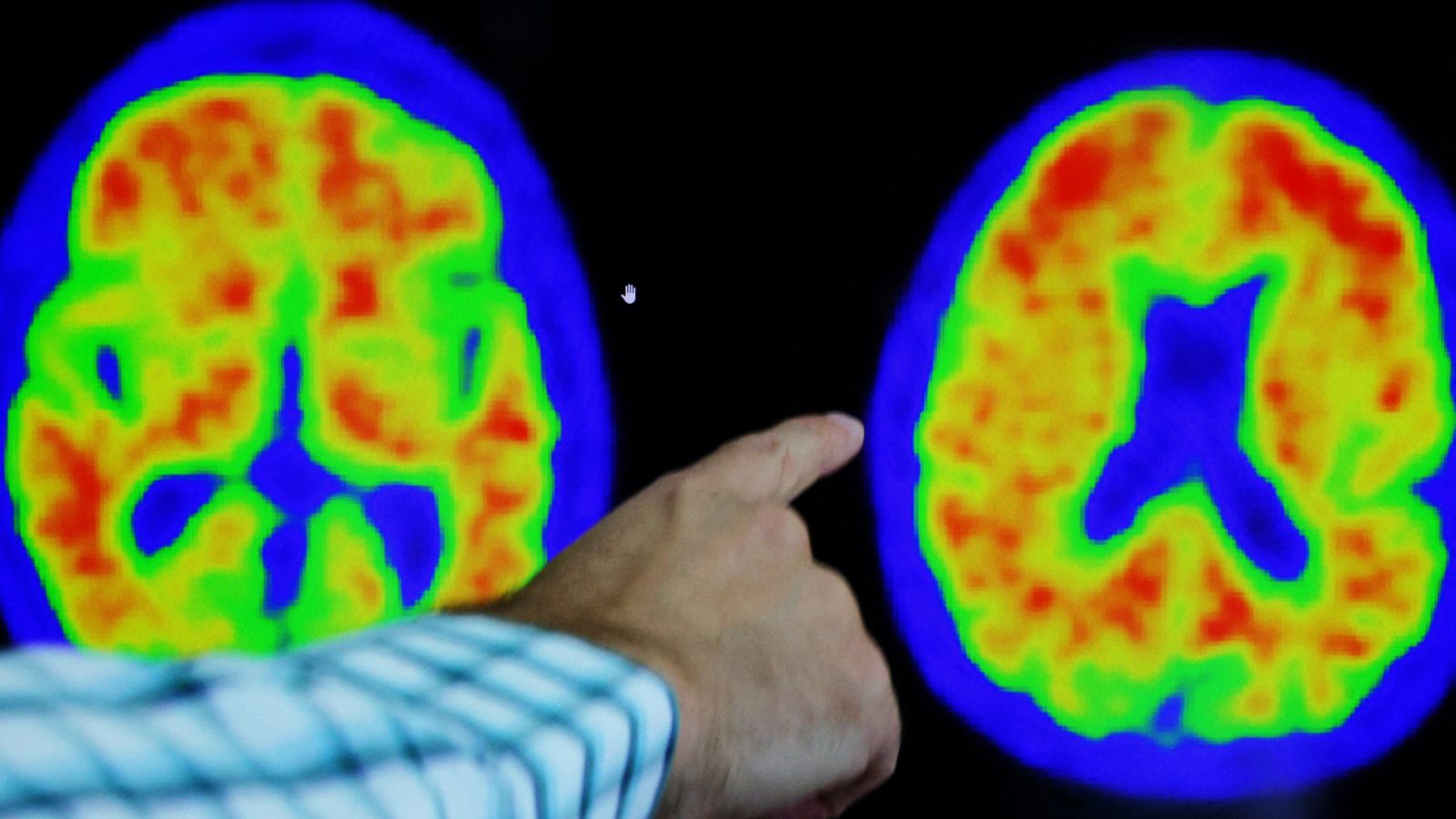
Donanemab works by removing plaques of a protein called amyloid that build up in the brain of people with Alzheimer’s.
Scientists have published the final results of a trial, known as TRAILBLAZER ALZ-2, examining the safety and efficacy of the drug, manufactured by US pharmaceutical giant Eli Lilly.
Researchers examined almost 1,800 people with early-stage Alzheimer’s, with half given a monthly infusion of donanemab into the bloodstream and the other half given a placebo over 18 months.
The study concluded, after 76 weeks of treatment, donanemab slowed clinical decline by 35.1% in people with early Alzheimer’s whose brain scans showed low or medium levels of a protein called tau.
When the results were combined for people who had different levels of this protein, there was a 22.3% slowing in disease progression.
Be the first to get Breaking News
Install the Sky News app for free
Side effects
The researchers found among a small number of people there were some serious side effects such as brain swelling.
Meanwhile, three deaths in the donanemab group and one in the placebo group were considered “treatment related”.
The findings were published in the Journal of the American Medical Association and presented to the Alzheimer’s Association International Conference in Amsterdam.
Eli Lilly said some taking the drug would be able to finish the course of treatment in six months once their amyloid plaque cleared.
It said treatment with donanemab reduced amyloid plaque on average by 84% at 18 months, compared with a 1% decrease for participants given a placebo.
Some 47% taking the drug who had early-stage disease and low or medium levels of tau were found to stall the disease for a year.
It comes after trials showed another drug called lecanemab slowed progression of Alzheimer’s symptoms by 27% in patients in the early stages of the disease. The drug was approved for use in the US earlier this month.
Science correspondent
The NHS is nowhere near ready to provide the first effective Alzheimer’s drugs to the huge numbers of people who need them.
Estimates by Alzheimer’s Research UK suggest 720,000 people in the UK would meet the treatment criteria used in the clinical trials of lecanemab and donanemab.
These drugs work best when given at the very first stages of Alzheimer’s, or earlier still when patients have what doctors call mild cognitive impairment.
But that needs a diagnosis the NHS just can’t deliver at the scale needed.
Read Thomas Moore’s analysis here.
‘It is possible to slow down the disease’
Dr Mark Mintun, group vice president of neuroscience research and development at Eli Lilly and president of Avid Radiopharmaceuticals, said: “People living with early, symptomatic Alzheimer’s disease are still working, enjoying trips, sharing quality time with family – they want to feel like themselves, for longer.
“The results of this study reinforce the importance of diagnosing and treating disease sooner than we do today.”
Read more:
TV presenter reveals she has Alzheimer’s at age of 62
Bruce Willis’s family praised after star’s diagnosis
Please use Chrome browser for a more accessible video player
Dr Richard Oakley, associate director of research at Alzheimer’s Society, said: “This is truly a turning point in the fight against Alzheimer’s and science is proving that it is possible to slow down the disease.
“Treatments like donanemab are the first steps towards a future where Alzheimer’s disease could be considered a long-term condition alongside diabetes or asthma – people may have to live with it, but they could have treatments that allow them to effectively manage their symptoms and continue to live fulfilled lives.”
Click to subscribe to the Sky News Daily wherever you get your podcasts
He added: “Diagnosis will be key to the access of any new treatments.
“We can’t have a situation where treatments are approved for use in the UK but people aren’t diagnosed early or accurately enough to be eligible.
“We need early, and accurate, diagnoses available for everyone and the NHS ready to roll out treatments such as donanemab and lecanemab if and when they are approved in the UK.”