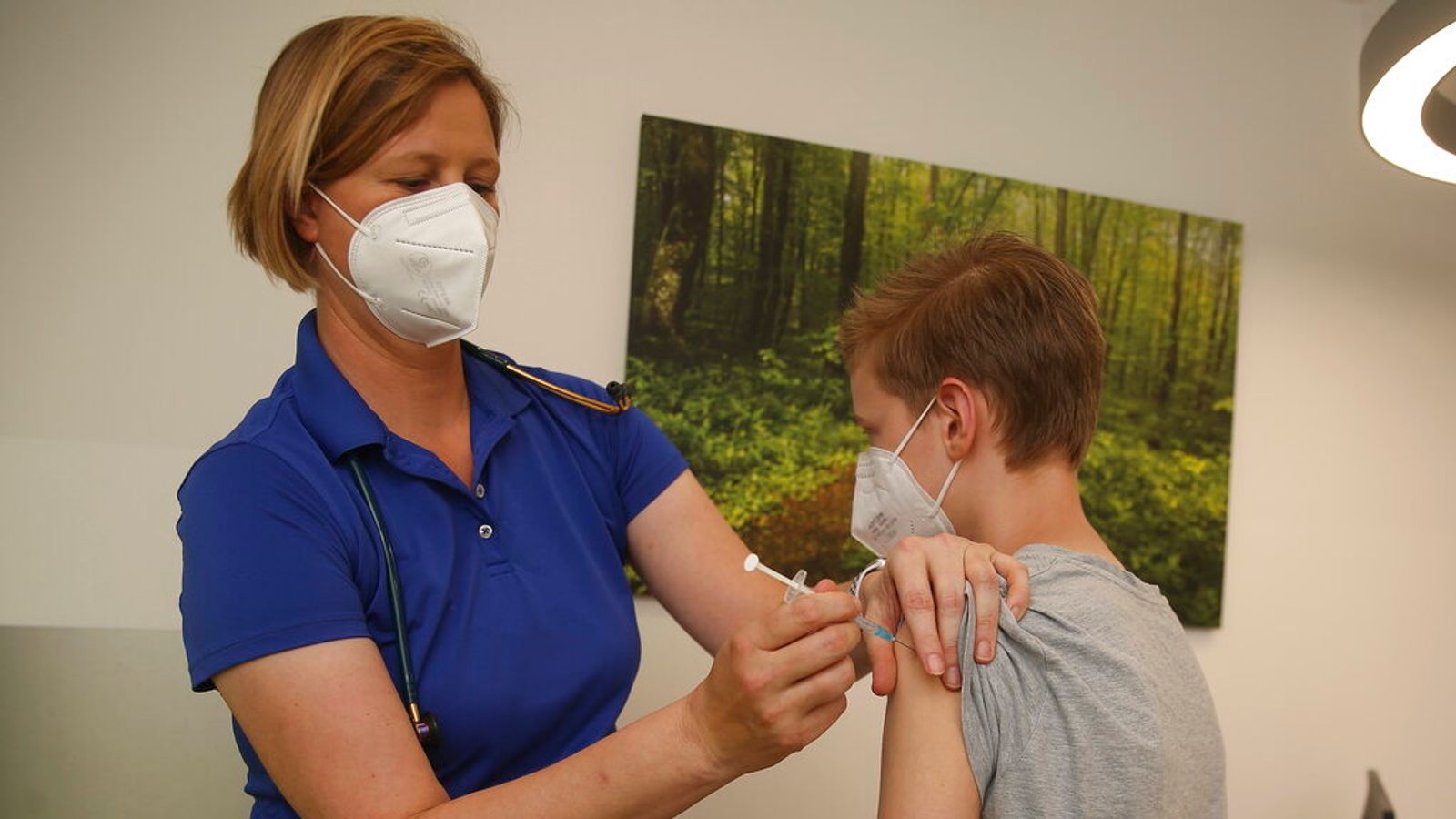
All 16 and 17-year-olds are to be offered a first dose of a coronavirus vaccine, the Joint Committee on Vaccination and Immunisation (JCVI) has recommended.
The UK will now follow other countries including the United States, Israel and France who have started to vaccinate older teenagers against COVID-19.
Live COVID updates from the UK and around the world
Dr June Raine, chief executive of the Medicines and Healthcare products Regulatory Agency (MHRA), said the decision had been taken after “rigorously reviewed” trials in children and young people.
She told a Downing Street briefing that the MHRA will “continue to scrutinise” the data as the first wave of teenagers come forward to get their jabs.
A second dose for this age group will be recommended after emerging safety data has been scrutinised, the government health advisory body said.
The first inoculations for about 1.4 million older teenagers will be offered in the next few weeks ahead of a return to classrooms for the start of the autumn term and children will not need the consent of their parents to get a jab.
Official close to the vaccination programme have said that under current guidance, if a child is able to understand the risks and benefits of any medical treatment then they can give their consent without the say-so of their parents.
Health Secretary Sajid Javid said he had accepted the JCVI’s recommendations and asked the NHS to prepare to vaccinate those eligible “as soon as possible”.
Boris Johnson said families should listen to the advice of the experts when it comes to COVID vaccination for children.
Please use Chrome browser for a more accessible video player
“I would just urge all families thinking about this across the country to listen to the JCVI,” the prime minister said.
“They are extremely expert there, they’re amongst the best if not the best in the world, they know what’s safe and I think we should listen to them and take our lead from them.”
Professor Wei Shen Lim, COVID-19 chair for the JCVI, said: “While COVID-19 is typically mild or asymptomatic in most young people, it can be very unpleasant for some and for this particular age group, we expect one dose of the vaccine to provide good protection against severe illness and hospitalisation.”
Younger children aged 12 to 15 will not be advised to get vaccinated in this phase but that could change later, with government scientists continuing to analyse data and evaluate any risks.
In July the JCVI said 12 to 15-year-olds who have an underlying health condition that put them at risk of severe COVID will be offered a vaccination.
And children aged 12 to 15 and live with or are close family contacts with someone who is deemed at risk should also be offered a vaccination. This advice has not changed.
But there has been significant debate over whether younger individuals should be offered the jab.
Some scientists say it would prevent further disruption to schooling in the next academic year, but other individuals have suggested that – as children are at a lower risk of serious illness from the virus – it would not be beneficial.
It was announced last month that clinically vulnerable children and those living with at-risk adults would be offered a vaccine.
The JCVI recommended that children “at an increased risk of serious COVID-19 disease” should be offered a jab.
As a result, children aged between 12 and 15-years-old with severe neurodisabilities, Down’s syndrome, immunosuppression and multiple or severe learning disabilities are being offered the Pfizer-BioNTech vaccine.
Children in the same age range who live with an immunosuppressed person are also being offered a vaccine, along with healthy children who are less than three months away from their 18th birthday.