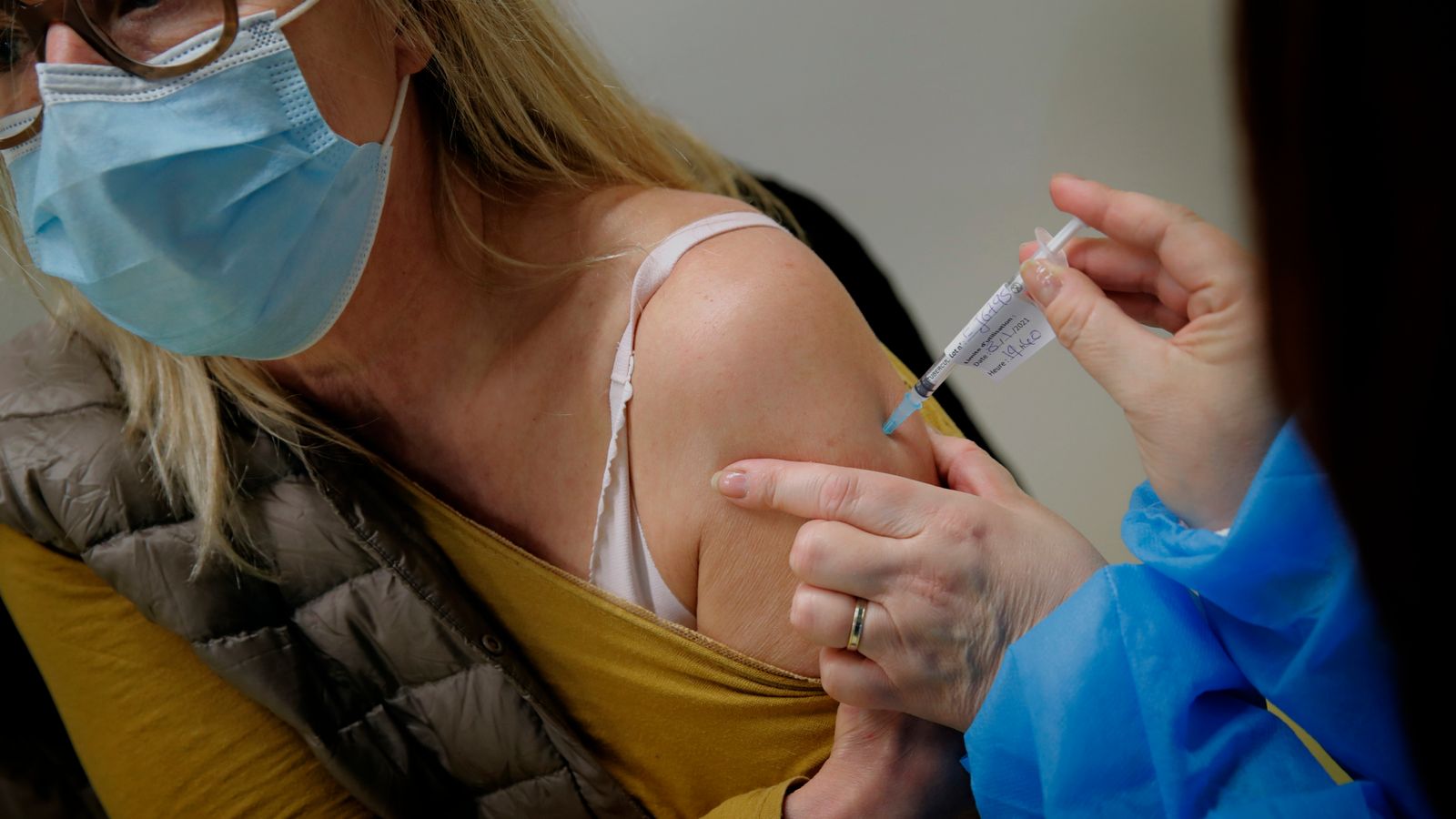
Vaccine clinical trial participants will be given the opportunity to receive two additional doses of an approved vaccine so they can travel abroad, the government has announced.
The majority of foreign countries currently do not recognise clinical trial volunteers as being fully vaccinated, instead requiring visitors to be jabbed with a COVID vaccine that is approved by regulators.
The additional doses will initially be offered to those taking part in the Novavax trial, before being rolled out to participants in other relevant trials within the coming weeks.
They will be offered two doses of the Pfizer/BioNTech vaccine from next week, with an eight-week interval between first and second doses.
Latest coronavirus updates from the UK and around the world
This new approach, which will apply to England, has been developed with the independent experts on the JCVI and the chief investigators for the clinical trials.
Deputy chief medical officer Professor Jonathan Van-Tam said: “The measures we have taken will allow UK COVID-19 vaccine trial participants to travel freely overseas once they have had the additional vaccinations. Those volunteers now have the flexibility to make a decision for themselves so they can, for example, visit loved ones abroad.
Coronavirus: Northern Ireland axes social distancing in bars and restaurants and allows nightclubs to reopen
COVID-19: NHS staff just as worried about pressures now as during peak of pandemic
Real wages effectively flatlined in lost decade of growth – but there’s a glimmer of light
“We should be very clear that the results from these trials benefit the whole world, and it has to be said that if more countries around the world had reciprocated by allowing UK volunteers to enjoy fully vaccinated status for overseas travel, these measures would not have been necessary.”
There are around 52,000 people currently taking part in trials across the UK, with 21,000 given a vaccine not yet approved for deployment by the Medicines and Healthcare products Regulatory Agency. Around 15,000 of these have taken part trials for the Novavax vaccine at 35 UK sites.
The UK has ordered 60 million doses of the Novavax vaccine, which was developed in the US, but will be produced in part by Fujifilm Diosynth Biotechnologies at its facility in Billingham on Teesside.
Letters will be sent out to clinical trial participants shortly, outlining further details and the next steps. Vaccinations will most likely take place at hospital hubs.
Principle investigator of the Novavax clinical trial Professor Paul Heath said: “For too long the participants have been disadvantaged in terms of international travel because this vaccine is not yet approved for deployment – but trial participants now have the flexibility to receive booster doses, or additional doses for travel purposes, if they wish to.”
People who have received both doses of a vaccine as part of a clinical trial will also be offered a booster jab, if eligible, in line with the wider boosters advice from the Joint Committee on Vaccination and Immunisation (JCVI).
This new approach, which will apply to England, has been developed with the independent experts on the JCVI and the chief investigators for the clinical trials.
The UK already recognises trial participants as fully vaccinated and they are able to use the NHS COVID Pass for domestic purposes.
The Department of Health and Social Care says it is working on a longer-term solution for trial participants looking to travel abroad with other countries through groups such as the G7, the EU Commission and the World Health Organisation (WHO).
Meanwhile, the travel red list is set to be slashed to seven countries, the government announced yesterday.
As of 4am on Monday, 47 countries will be removed from the red list so travellers arriving from those destinations will no longer have to quarantine in a government-approved hotel for 11 nights at a cost of £2,285.
The only countries remaining on the red list are Panama, Colombia, Venezuela, Peru, Ecuador, Haiti and the Dominican Republic.