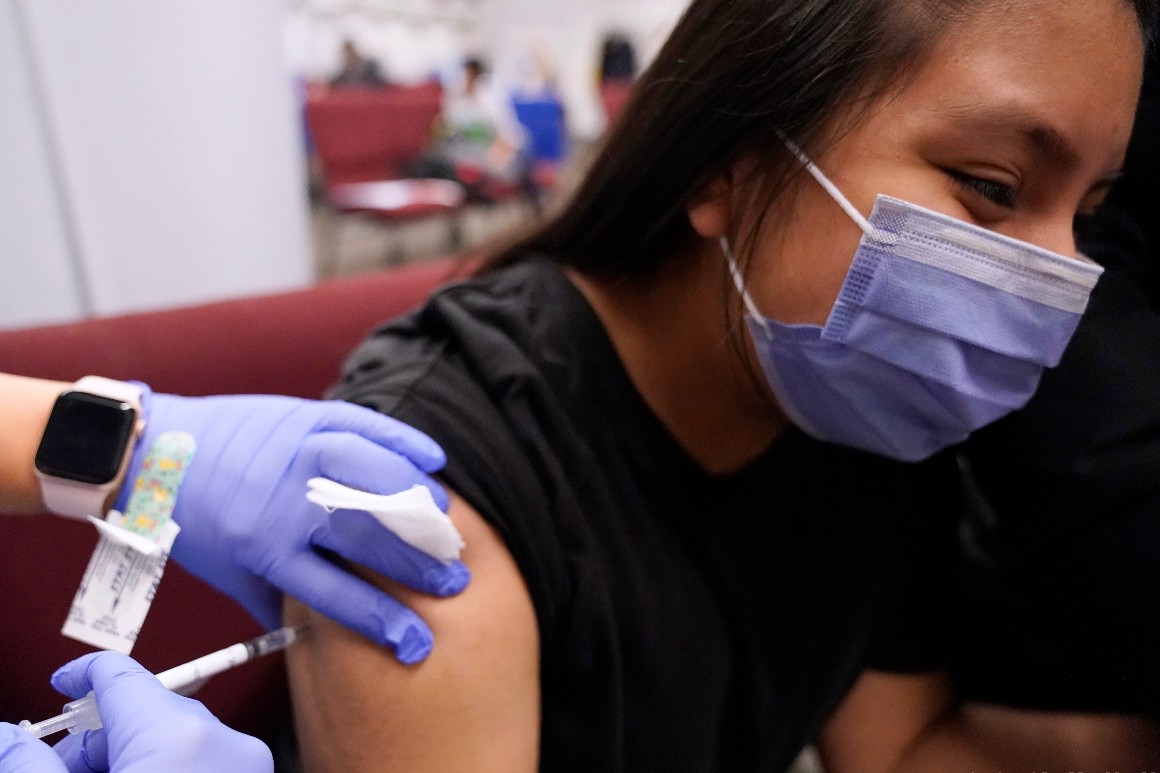
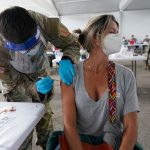
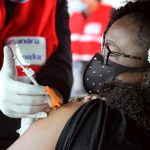
The companies submitted Phase III clinical trial data to FDA as a supplemental application for their vaccine, which is already approved for adults and children as young as 16.
- Covid-19 / Coronavirus stats for United States of America (USA)
- Covid-19 / Coronavirus stats for Canada
- Covid-19 / Coronavirus stats for Italy
- Covid-19 / Coronavirus stats for Spain
- Covid-19 / Coronavirus stats for China
- Covid-19 / Coronavirus stats for India
- Covid-19 / Coronavirus Confirmed cases Worldwide
- Covid-19 / Coronavirus dealth cases Worldwide
- Covid-19 / Coronavirus stats for United Kingdom (UK)
- Covid-19 / Coronavirus stats for Iran
- Covid-19 / Coronavirus stats for Iraq
- Covid-19 / Coronavirus stats for Switzerland
- AI Gathered Information About Covid-19
- Covid-19 / Coronavirus Recovered cases Worldwide