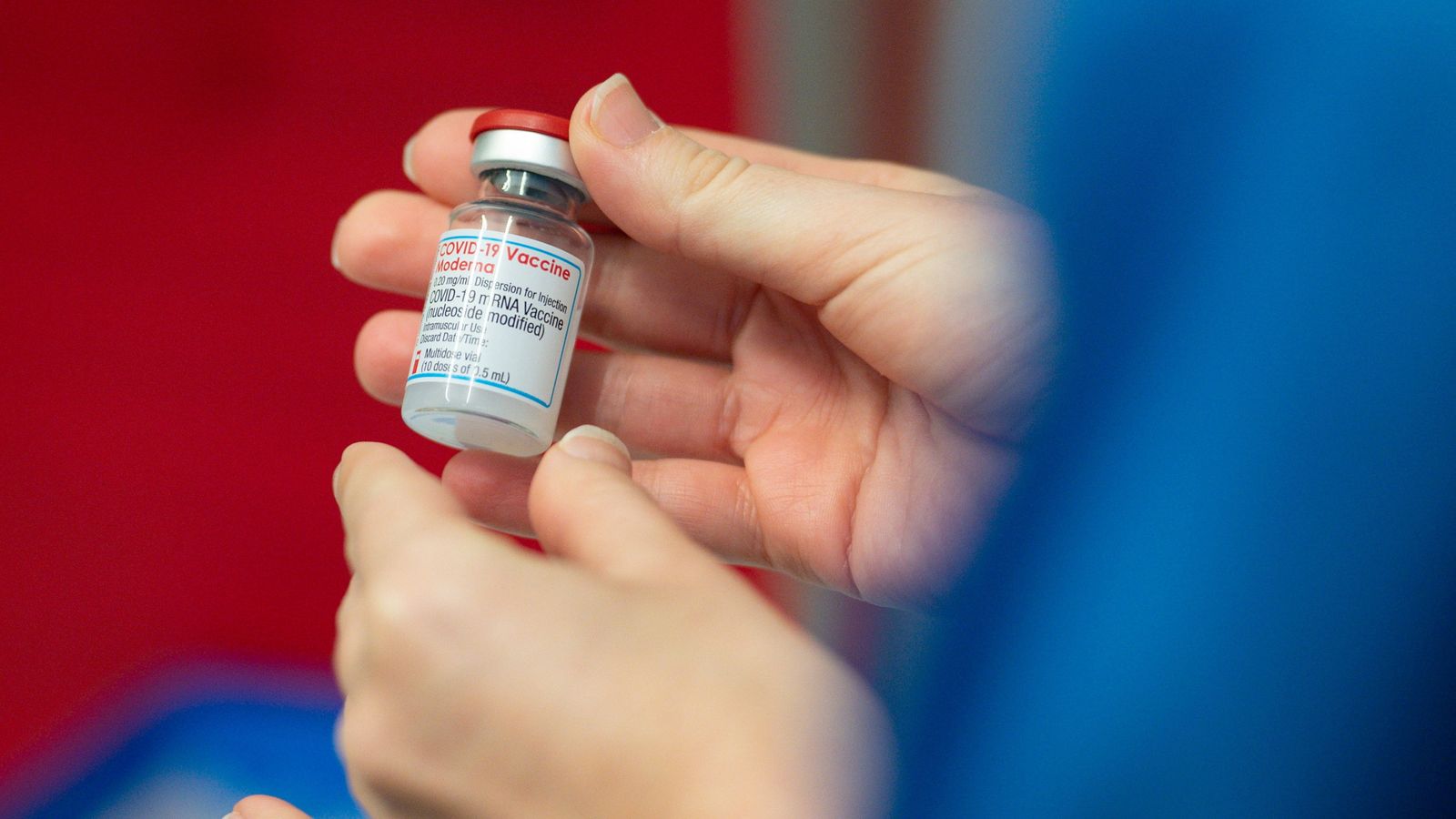
The UK health regulator has approved the Moderna coronavirus vaccine for 12 to 17-year-olds.
The Medicines and Healthcare products Regulatory Agency (MHRA) said it is now up to the Joint Committee on Vaccination and Immunisation (JCVI) to advise the government on whether children in this age group should be given the jab.
Dr June Raine, MHRA chief executive, said: “I am pleased to confirm that that the COVID-19 vaccine made by Moderna has now been authorised in 12 to 17-year-olds. The vaccine is safe and effective in this age group.
“We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved COVID-19 vaccines and this surveillance will include the 12 to 17-year age group.
“It is for the Joint Committee on Vaccination and Immunisation (JCVI) to advise on whether this age group should be vaccinated with the Covid-19 vaccine made by Moderna as part of the deployment programme.”
All teenagers aged 16 and 17 in the UK are to be offered a coronavirus vaccine by the 23 August.
Please use Chrome browser for a more accessible video player
Health Secretary Sajid Javid said the target date will allow the nation’s 1.4 million teenagers to get “vital protection” before returning to sixth form or college two weeks later in September.
The vaccine is already available to children aged 12 and over if their health leaves them at higher risk, or if they live with an immunosuppressed person.
According to the Department of Health and Social Care (DHSC), children in this group will be invited for their vaccine by 23 August.
The DHSC also said that some 100,000 text messages are also being sent to teenagers within three months of turning 18, inviting them to book their vaccine appointment online through the National Booking Service or by calling 119.
On the Moderna vaccine being approved, a spokesperson for the department said: “We welcome the news that Moderna’s vaccine has been approved as safe and effective for people aged 12 and over.
“As has been the case with all other approvals, we will now be guided by the independent Joint Committee on Vaccination and Immunisation (JCVI) and have asked for its formal recommendation on whether to administer this vaccine to people aged 12 to 17.”
The approval comes more than two months after the Pfizer-BioNTech jab got the greenlight use in children aged 12 to 15 in the UK. It was already approved for use in over-16s.
Moderna is already authorised for children aged 12-17 years in Northern Ireland. Those aged 12-15 were invited for a jab in The Republic of Ireland from Saturday, with groups of parents and children queuing up for a jab.
It was also recommended for use in adolescents by European regulators in July but is awaiting US authorisation.
The Republic of Ireland joins the likes of the United States, Israel, France and a number of other nations who are already inoculating young people against coronavirus.