Two types of contraceptive pill will be available to buy without a prescription from pharmacies, the UK medicine regulator has ruled.
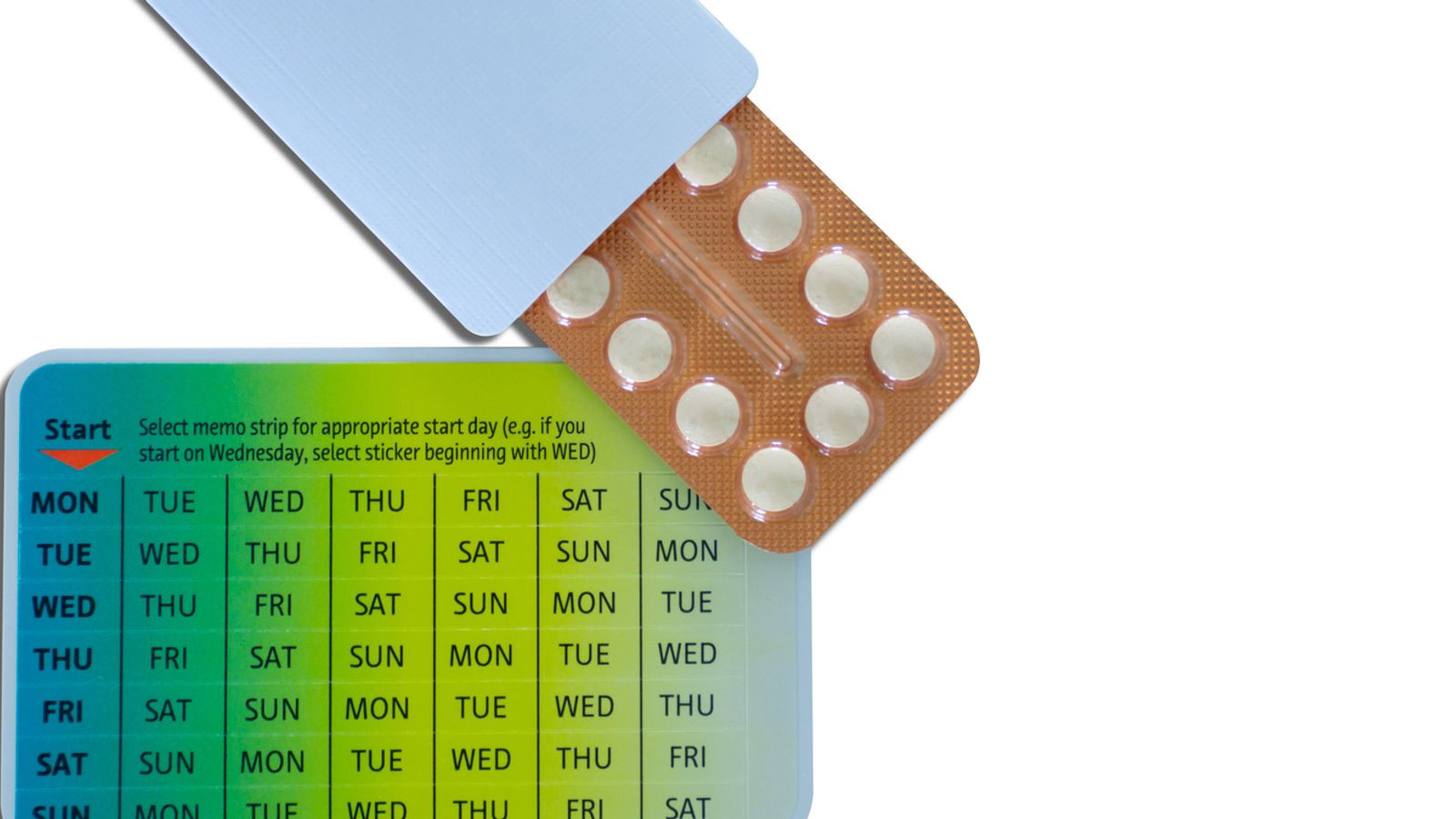
Progestogen-only Hana and Lovima tablets can now be provided over the counter after being reclassified by the Medicines and Healthcare products Regulatory Agency (MHRA).
It marks the first time women will be able to buy progesterone-only oral contraceptive pills, which contain desogestrel, without prescription in the UK.
However, purchasers will still need to have a consultation with the pharmacist.
MHRA says desogestrel is safe for most women to take and will still be available free of charge from a doctor, commissioned services and sexual health clinics.
Pills that combine oestrogen and progesterone will still require a prescription.
Dr June Raine CBE, chief executive of MHRA, said the ruling is “good news” for women and families.
She said: “Pharmacists have the expertise to advise women on whether desogestrel is an appropriate and safe oral contraceptive pill for them to use and to give women the information they need, to make informed choices.
“We have consulted a wide range of people to enable us to reach the decision to make this contraceptive available for the first time in the UK without prescription. We received many responses to our consultation, the majority of which supported this approach.”
Dr Edward Morris, president of the Royal College of Obstetricians and Gynaecologists (RCOG), said the announcement is a “huge win for women and girls” who will no longer face unnecessary barriers when accessing contraception.
He added: “Even before the pandemic, too many women and girls were struggling to access basic women’s health services.
“The consequences of this include an increase in the number of unplanned pregnancies, which can result in poorer outcomes for women and their babies.”
He added: “Enabling women and girls to access POP more easily and conveniently will give them more control over their reproductive health, which can only be a good thing.”
The tablets, Lovima 75 microgram film-coated tablets and Hana 75 microgram film-coated tablets, are both oral contraceptives for continuous use to prevent pregnancy in women of childbearing age.
The MHRA’s decision to reclassify the pills follows a safety review by the Commission on Human Medicines (CHM) and a public consultation.
The public consultation received 494 responses, with over 80% in favour of reclassifying the pills.